
Chemistry, 09.06.2021 03:00 blkrosesss
Calculate the number of milliliters of 0.587 M NaOH required to precipitate all of the Fe3 ions in 197 mL of 0.654 M FeCl3 solution as Fe(OH)3. The equation for the reaction is: FeCl3(aq) 3NaOH(aq) Fe(OH)3(s) 3NaCl(aq)

Answers: 2
Another question on Chemistry


Chemistry, 23.06.2019 08:00
Which of the following notations would be the appropriate and final way to display the formula for magnesium chloride a. mgcl2 b. mg+2cl–1 c. mgcl2 d. mgcl
Answers: 2

Chemistry, 23.06.2019 11:00
Acompound is isolated from the rind of lemons that is found to be 88.14% carbon and 11.86% hydrogen by mass how many grams of c and h?
Answers: 2

Chemistry, 23.06.2019 12:50
How many energy levels contain electrons in an atom of zirconium (zr)?
Answers: 1
You know the right answer?
Calculate the number of milliliters of 0.587 M NaOH required to precipitate all of the Fe3 ions in 1...
Questions

History, 30.12.2019 21:31


Mathematics, 30.12.2019 21:31

Biology, 30.12.2019 21:31



Chemistry, 30.12.2019 21:31

Mathematics, 30.12.2019 21:31


History, 30.12.2019 21:31

Mathematics, 30.12.2019 21:31

Mathematics, 30.12.2019 21:31

Mathematics, 30.12.2019 21:31

Mathematics, 30.12.2019 21:31




Mathematics, 30.12.2019 21:31

Mathematics, 30.12.2019 21:31

Geography, 30.12.2019 21:31

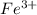 ions in 197 mL of 0.654 M
ions in 197 mL of 0.654 M 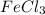 solution as
solution as 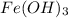 .
.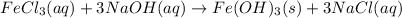
 Volume (in L)
Volume (in L)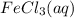 reacts with 3 moles of NaOH(aq). Hence, moles of
reacts with 3 moles of NaOH(aq). Hence, moles of 

