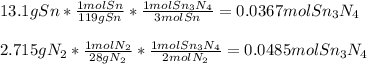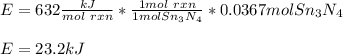How much energy is produced when 13.1 g of tin reacts with 2.715 g of N2 ?
I.
3 Sn + 2 N2Sn3N...

Chemistry, 09.06.2021 07:50 matthewlucas8613
How much energy is produced when 13.1 g of tin reacts with 2.715 g of N2 ?
I.
3 Sn + 2 N2Sn3N4 + 632 KJ
Hint change grams to moles first.
1 mole Sn= 119g
1 mole N2= 28 g

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:10
When the volume and number of particles of a gas are constant which of the following is also constant
Answers: 3

Chemistry, 22.06.2019 09:50
What are four significant sources of ghgs that come from wostem washington?
Answers: 2


You know the right answer?
Questions




Geography, 10.11.2020 17:00






Mathematics, 10.11.2020 17:00






English, 10.11.2020 17:00

World Languages, 10.11.2020 17:00

History, 10.11.2020 17:00

Computers and Technology, 10.11.2020 17:00