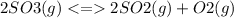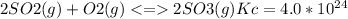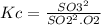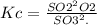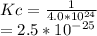Chemistry, 09.06.2021 14:00 slawson4328
The value for the equilibrium constant for the reaction 2SO2(g)+O2(g) rightleftharpoons 2SO3(g) is 4.0*10^ 1 24 at 298K What would be the value for the equilibrium constant for the following reaction at the same temperature? 2SO3(g) rightleftharpoons2502(g)+O2(g)

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:00
Which object forms when a supergiant runs out of fuel? a red giant a black hole a white dwarf a neutron star
Answers: 1

Chemistry, 22.06.2019 04:00
The continuous release of nuclear energy caused when one fission reaction triggered more nuclear reactions is a
Answers: 3

Chemistry, 22.06.2019 06:30
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1

You know the right answer?
The value for the equilibrium constant for the reaction 2SO2(g)+O2(g) rightleftharpoons 2SO3(g) is 4...
Questions

Social Studies, 10.01.2021 19:40

Mathematics, 10.01.2021 19:40

Biology, 10.01.2021 19:40

Arts, 10.01.2021 19:40


Mathematics, 10.01.2021 19:40


Biology, 10.01.2021 19:40

Mathematics, 10.01.2021 19:40

English, 10.01.2021 19:40



History, 10.01.2021 19:40

Social Studies, 10.01.2021 19:40


Mathematics, 10.01.2021 19:40



Mathematics, 10.01.2021 19:40

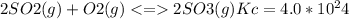 at 298K .
at 298K .