
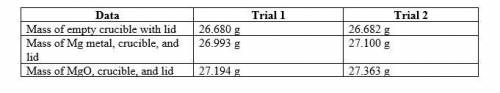
The chemical equation is Mg(s) + O2(g) → MgO(s)
1. Magnesium is the limiting reactant in this experiment. What is the theoretical yield of MgO for each trial?
2. what is the percent yield of MgO for the experiment for each trial?
3. what is the average percent yield of MgO for the two trials?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 10:10
What shape would a molecule with two bound groups and two lone pairs have?
Answers: 1


Chemistry, 22.06.2019 12:00
What is the subscript for oxygen in its molecular formula
Answers: 1

You know the right answer?
The chemical equation is Mg(s) + O2(g) → MgO(s)
1. Magnesium is the limiting reactant in this exper...
Questions




Chemistry, 02.12.2020 05:00


Mathematics, 02.12.2020 05:00


Mathematics, 02.12.2020 05:00

Mathematics, 02.12.2020 05:10

Mathematics, 02.12.2020 05:10

Chemistry, 02.12.2020 05:10


History, 02.12.2020 05:10



Mathematics, 02.12.2020 05:10


Mathematics, 02.12.2020 05:10


Mathematics, 02.12.2020 05:10



