
HELP!!
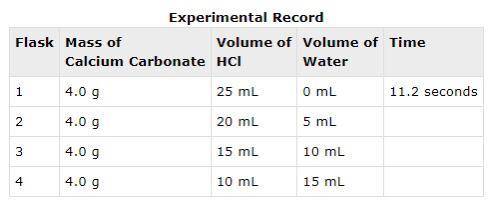
In an experiment, calcium carbonate reacted with different volumes of hydrochloric acid in water. One of the products formed during the experiment was carbon dioxide. The time taken for 0.89 mL of carbon dioxide to form was recorded. A partial record of the experiment is shown.
Based on your knowledge of factors that affect the rates of chemical reactions, predict the trend in the last column of the experimental record. Use complete sentences to explain the trend you predicted. You do not have to determine exact values for time; just describe the trend you would expect (increase or decrease) and why it occurs.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 13:00
Drag each tile to the correct box arrange the layers not order from oldest to youngest
Answers: 2

Chemistry, 21.06.2019 17:30
Which type of stress results when two plates push against one another? a. compression b. tension c. force d. shear
Answers: 1

Chemistry, 22.06.2019 13:30
Apush or pull that moves or changes and object when to objects touch
Answers: 2

Chemistry, 22.06.2019 15:30
Draw the lewis dot structure for each of the following polyatomic ions
Answers: 1
You know the right answer?
HELP!!
In an experiment, calcium carbonate reacted with different volumes of hydrochloric acid in w...
Questions

Mathematics, 19.04.2020 21:22

Mathematics, 19.04.2020 21:22


Physics, 19.04.2020 21:23




Mathematics, 19.04.2020 21:23

Health, 19.04.2020 21:23

English, 19.04.2020 21:23

Physics, 19.04.2020 21:23

Biology, 19.04.2020 21:23


History, 19.04.2020 21:24



Spanish, 19.04.2020 21:24


Mathematics, 19.04.2020 21:24

Mathematics, 19.04.2020 21:24



