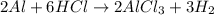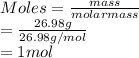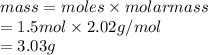Chemistry, 10.06.2021 15:20 drandbone92
Calculate the mass of hydrogen formed when 26.98 g of aluminum reacts with excess hydrochloric acid according to the following balanced chemical equation: 2 Al + 6 HCl → 2 AlCl3 + 3 H2

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 19:30
Si una estrella no tiene paralaje medible, ¿qué puedes inferir?
Answers: 1

Chemistry, 22.06.2019 08:00
Define dew point. i am writing this part to be able to ask the question
Answers: 1

Chemistry, 22.06.2019 11:50
The chemical bond connecting one nucleotide with the next one along the nucleic acid chain is called a
Answers: 3

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 3
You know the right answer?
Calculate the mass of hydrogen formed when 26.98 g of aluminum reacts with excess hydrochloric acid...
Questions


Mathematics, 23.03.2021 19:50



Mathematics, 23.03.2021 19:50

History, 23.03.2021 19:50

Social Studies, 23.03.2021 19:50



Chemistry, 23.03.2021 19:50

Mathematics, 23.03.2021 19:50


Mathematics, 23.03.2021 19:50


Mathematics, 23.03.2021 19:50

Chemistry, 23.03.2021 19:50

Mathematics, 23.03.2021 19:50



Mathematics, 23.03.2021 19:50