
Chemistry, 10.06.2021 21:50 vanvalenpeyt
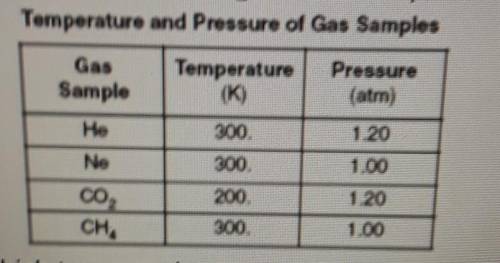
The day table below gives the temperature and pressure of four different gas samples, each in a 2-liter container.
Which two samples contain the same total number of particles?
A) CHA and CO2
B) CH4 and Ne
C) He and CO2
D) He and Ne

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 20:00
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2

Chemistry, 22.06.2019 21:30
How can the periodic table be used to predict the behavior of elements?
Answers: 1

Chemistry, 22.06.2019 22:30
Which process describes vaporization that takes place below the surface of a liquid? condensation melting boiling evaporation
Answers: 1

Chemistry, 22.06.2019 23:00
How does the value of the equilibrium constant show that a reaction reaches equilibrium very quickly? (a) the equilibrium constant is large. (b) the equilibrium constant is small. (c) the equilibrium constant is zero. (d) the value of the equilibrium constant does not show how quickly a reaction comes to equilibrium.
Answers: 1
You know the right answer?
The day table below gives the temperature and pressure of four different gas samples, each in a 2-li...
Questions






Mathematics, 08.02.2022 02:40


Mathematics, 08.02.2022 02:50

Mathematics, 08.02.2022 02:50



History, 08.02.2022 02:50


English, 08.02.2022 02:50

Mathematics, 08.02.2022 02:50




Mathematics, 08.02.2022 02:50

Biology, 08.02.2022 02:50



