
Chemistry, 10.06.2021 23:20 jonathon3957
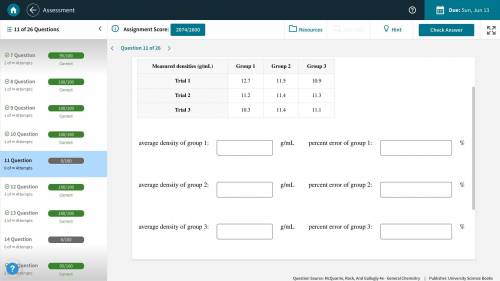
(Picture Included) A handbook lists the density of lead as 11.3 g/mL. Several groups of students are attempting to determine the density of a lead weight by various methods. Calculate the average density measured by each group, and the percentage error in each average.

Answers: 1
Another question on Chemistry



Chemistry, 22.06.2019 10:10
How do you identify the anode on a power source such as a battery? how do you identify the cathode? how are terms anion and cation?
Answers: 1

Chemistry, 22.06.2019 10:20
In a reaction equation, where are the products located? a.) above the arrow b.) to the right of the arrow c.) to the left of the arrow d.) below the arrow
Answers: 2
You know the right answer?
(Picture Included) A handbook lists the density of lead as 11.3 g/mL. Several groups of students are...
Questions

Biology, 03.10.2019 07:30

Mathematics, 03.10.2019 07:30


English, 03.10.2019 07:30

Mathematics, 03.10.2019 07:30


English, 03.10.2019 07:30

Computers and Technology, 03.10.2019 07:30

Mathematics, 03.10.2019 07:30


Mathematics, 03.10.2019 07:30



Mathematics, 03.10.2019 07:30

Mathematics, 03.10.2019 07:30


Mathematics, 03.10.2019 07:30


Mathematics, 03.10.2019 07:30



