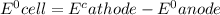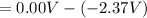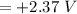Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 20:30
Calculate the percent composition by mass of each element in al(oh)3. use at least three significant figures.
Answers: 1

Chemistry, 22.06.2019 23:00
What is the most common reason for matter changing its state?
Answers: 1

Chemistry, 22.06.2019 23:30
Match each statement with the state of matter it describes
Answers: 3

You know the right answer?
A voltaic cell consists of a standard hydrogen electrode and a second half-cell in which a magnesium...
Questions

Chemistry, 25.01.2021 06:40


Computers and Technology, 25.01.2021 06:40

Mathematics, 25.01.2021 06:40


Mathematics, 25.01.2021 06:40

Mathematics, 25.01.2021 06:40




Mathematics, 25.01.2021 06:40

Mathematics, 25.01.2021 06:40

Physics, 25.01.2021 06:40

Spanish, 25.01.2021 06:40

English, 25.01.2021 06:40


Mathematics, 25.01.2021 06:40


Chemistry, 25.01.2021 06:40

Mathematics, 25.01.2021 06:40

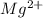
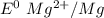 ,
,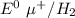 ,
,