
Chemistry, 11.06.2021 02:00 alydiale584
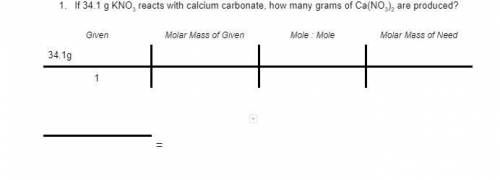
2KNO3 + CaCO3 → K2CO3 + Ca(NO3)2
If 34.1 g KNO3 reacts with calcium carbonate, how many grams of Ca(NO3)2 are produced?
1

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 10:00
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2


Chemistry, 22.06.2019 16:00
Inside a flashbulb, oxygen surrounds a thin coil of magnesium. when the flashbulb is set off, a chemical reaction takes place in which magnesium combines with oxygen to form magnesium oxide. which of the chemical equations matches the reaction above? a. mg + o2 mgo2 + energy b. 2mg + o mg2o + energy c. 2mg + o2 2mgo + energy d. mg + o mgo + energy
Answers: 1
You know the right answer?
2KNO3 + CaCO3 → K2CO3 + Ca(NO3)2
If 34.1 g KNO3 reacts with calcium carbonate, how many grams of Ca...
Questions

History, 27.06.2019 07:30





Mathematics, 27.06.2019 07:30






Mathematics, 27.06.2019 07:30

Advanced Placement (AP), 27.06.2019 07:30



Mathematics, 27.06.2019 07:30

History, 27.06.2019 07:30


Spanish, 27.06.2019 07:30



