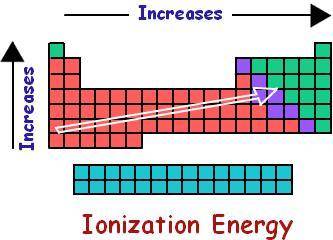
The first ionization energy of an element is the energy required to remove an electron from a gaseous atom of an element to produce a +1 ion:
M(g) + energy ---> M ^(+) (g) + e -
How do you think the activity of an element ought to be related to its first ionization energy? Predict a decreasing order of reactivity of the above elements based on their first ionization energies.

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 09:00
Look at the spectrums of a star moving towards earth and a motionless star. which of these is a correct inference that can be draw from the observation of the two spectrums? (2 points) the spectrum of a motionless star is difficult to be viewed separately using oridinary telescopes. the spectrum of a motionless star is identical to the spectrum of a star which moves towards earth. the spectrum of a star shifts towards the red region when the star moves towards earth. the spectrum of a star shifts towards the blue region when the star moves towards earth.
Answers: 2

Chemistry, 22.06.2019 14:30
What type(s) of intermolecular forces are expected between ch3ch2cooh molecules? dipole forces, induced dipole forces, hydrogen bonding
Answers: 1

Chemistry, 22.06.2019 18:30
Which of the following words describe the reality that the universe looks the same from various perspective
Answers: 3
You know the right answer?
The first ionization energy of an element is the energy required to remove an electron from a gaseou...
Questions



History, 18.07.2019 02:30


Mathematics, 18.07.2019 02:30


Mathematics, 18.07.2019 02:30



Mathematics, 18.07.2019 02:30

Mathematics, 18.07.2019 02:30


History, 18.07.2019 02:30

Mathematics, 18.07.2019 02:30



Mathematics, 18.07.2019 02:30

Arts, 18.07.2019 02:30

Chemistry, 18.07.2019 02:30