Chemistry, 11.06.2021 06:50 smcardenas02
What is the delta H when 72.0 grams H2O condenses at 100.00C?
Here are some constants that you MAY need.
specific heats heat of fusion heat of vaporization
H2O(s) = 2.1 J/g0C 6.01 kJ/mole 40.7 kJ/mole
H2O(L) = 4.18 J/g0C
H2O(g) = 1.7 J/g0C
2930 kJ
163 kJ
-163 kJ
-2930 kJ

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 12:30
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2

Chemistry, 22.06.2019 19:00
Structure of the atoms: discovery of the nucleus in 1909i need answering all of these questions
Answers: 3

Chemistry, 22.06.2019 20:30
A40 kilogram skier starts at the top of a 12 meter high slope. at the bottom, she is travelling 10 meters per second. how much energy does she lose to friction
Answers: 2

Chemistry, 23.06.2019 08:00
Match the vocabulary terms to their definitions. 1 . a long, chain-like set of molecules made up of repeating units joined end to end polymer 2 . a hard, brittle, heat- and corrosion-resistant material made by subjecting a nonmetallic mineral mixture to intense heat ceramic 3 . a plastic with low elongations that cannot be recycled thermoset 4 . a carbon fiber embedded in a polymer resin matrix thermoplastic 5 . a plastic with high elongations that can be recycled crystal 6 . a solid form resulting from the arrangement of atoms, ions, or molecules in definite geometric patterns composite
Answers: 1
You know the right answer?
What is the delta H when 72.0 grams H2O condenses at 100.00C?
Here are some constants that you MAY...
Questions

Mathematics, 30.10.2020 18:10


Health, 30.10.2020 18:10

Mathematics, 30.10.2020 18:10



Computers and Technology, 30.10.2020 18:10

Mathematics, 30.10.2020 18:10

Mathematics, 30.10.2020 18:10

Social Studies, 30.10.2020 18:10





Chemistry, 30.10.2020 18:10

Mathematics, 30.10.2020 18:10


Mathematics, 30.10.2020 18:10


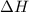 is -163 kJZ
is -163 kJZ ......(1)
......(1)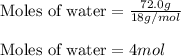
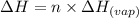 ......(2)
......(2)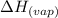 = specific heat of vaporization = -40.7 kJ/mol
= specific heat of vaporization = -40.7 kJ/mol