
Chemistry, 12.06.2021 01:40 benjaminmccutch
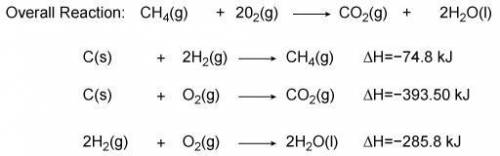
Use the information from the diagram to calculate the enthalpy of combustion for methane.
Question 2 options:
A)
+752 kJ
B)
–921 kJ
C)
–604 kJ
D)
+604 kJ

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:20
Much of the general structure and physical properties of the interior of the earth are inferred from: a)deep oil and gas bore holes b)geologic investigations c)analysis of seismic waves d) study of volcanoes
Answers: 1

Chemistry, 22.06.2019 13:30
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a.the mitochondria b.the nucleus c.the vacuoles d.the endoplasmic reticulum
Answers: 1

Chemistry, 22.06.2019 15:30
How does a large body of water, such as the ocean, influence climate?
Answers: 1

Chemistry, 22.06.2019 17:40
Which statement about hf is true? it is zero for any compound in its standard state. it is positive when the bonds of the product store more energy than those of the reactants. it is negative when a compound forms from elements in their standard states. it is zero for any element that is in the liquid state.
Answers: 1
You know the right answer?
Use the information from the diagram to calculate the enthalpy of combustion for methane.
Question...
Questions


Biology, 28.09.2019 16:30

History, 28.09.2019 16:30

History, 28.09.2019 16:30


History, 28.09.2019 16:30



History, 28.09.2019 16:30






Computers and Technology, 28.09.2019 16:30

Social Studies, 28.09.2019 16:30


History, 28.09.2019 16:30




