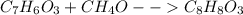Chemistry, 12.06.2021 02:10 kekoanabor19
Methyl salicylate (oil of wintergreen) is prepared by heating salicylic acid, , with methanol, . In an experiment, 1.60 g of salicylic acid is reacted with 15.0 g of methanol. The yield of methyl salicylate, , is 1.66 g. What is the percentage yield

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:30
1. explain hydrogen peroxide, h 2 o 2 properties and decomposition reaction. 2. describe how each of the following natural cycles plays a part in earth’s climate system. (a) the water cycle (b) the carbon cycle
Answers: 1

Chemistry, 22.06.2019 16:30
Ammonium perchlorate nh4clo4 is the solid rocket fuel used by the u.s. space shuttle. it reacts with itself to produce nitrogen gas n2 , chlorine gas cl2 , oxygen gas o2 , water h2o , and a great deal of energy. what mass of nitrogen gas is produced by the reaction of 2.1g of ammonium perchlorate?
Answers: 2

Chemistry, 22.06.2019 19:30
Which one of the following substances would be the most soluble in ccl4? na2so4 h2o ch3ch2ch2ch2oh c4h10 hi
Answers: 1

Chemistry, 22.06.2019 22:30
Which of the following molecules is polar? c3h7oh c2h5cooh
Answers: 1
You know the right answer?
Methyl salicylate (oil of wintergreen) is prepared by heating salicylic acid, , with methanol, . In...
Questions

History, 04.12.2020 02:40


German, 04.12.2020 02:40



History, 04.12.2020 02:40


Mathematics, 04.12.2020 02:40

Mathematics, 04.12.2020 02:40

Mathematics, 04.12.2020 02:40



Mathematics, 04.12.2020 02:40

Mathematics, 04.12.2020 02:40

Computers and Technology, 04.12.2020 02:40


Mathematics, 04.12.2020 02:40