Chemistry, 12.06.2021 02:30 addisonwiles
A buffer solution contains 0.496 M KHCO3 and 0.340 M K2CO3. If 0.0585 moles of potassium hydroxide are added to 250. mL of this buffer, what is the pH of the resulting solution

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1

Chemistry, 23.06.2019 03:00
A0.100-kilogram apple hangs in a tree 1.50 meter above the ground. ignore frictional effects, the total mechanical energy of the apples is
Answers: 1

Chemistry, 23.06.2019 07:30
Type the letter that represents the correct location for each particle type below. the neutron is found at __ the electron is found at __ the proton is found at __
Answers: 2

Chemistry, 23.06.2019 09:00
Describe the process that was used in this lab to create magnesium oxide, specifically identifying the type of chemical reaction. explain why the product had a higher mass than the reactant, and how this relates to conservation of matter.
Answers: 2
You know the right answer?
A buffer solution contains 0.496 M KHCO3 and 0.340 M K2CO3. If 0.0585 moles of potassium hydroxide a...
Questions

Mathematics, 29.09.2020 01:01


English, 29.09.2020 01:01

Mathematics, 29.09.2020 01:01

Mathematics, 29.09.2020 01:01

Chemistry, 29.09.2020 01:01





English, 29.09.2020 01:01

Mathematics, 29.09.2020 01:01



Chemistry, 29.09.2020 01:01

Mathematics, 29.09.2020 01:01


Biology, 29.09.2020 01:01


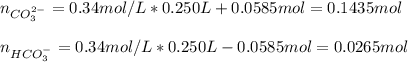
![[CO_3^{2-}]=\frac{0.1435mol}{0.25L}=0.574M \\](/tpl/images/1372/2885/db894.png)
![[HCO_3^{-}]=\frac{0.0265mol}{0.25L}=0.106M](/tpl/images/1372/2885/2de97.png)