
Chemistry, 12.06.2021 14:00 shetherealbrat
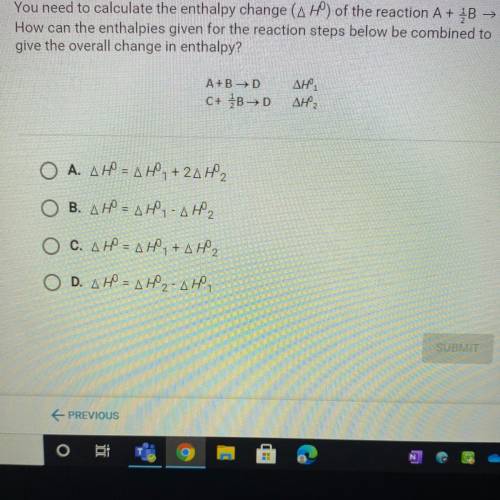
You need to calculate the enthalpy change (AH) of the reaction A + B → C.
How can the enthalpies given for the reaction steps below be combined to
give the overall change in enthalpy?
A+B →D
C+ B>D
ΔΗ,
ΔΗ,
Ο Α. ΔΗ = ΔΗ, + 2ΔΗ,
B. AH = AH - AH2
O C. AHN AH,+AH2
O D. AH = AH2-AH,

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
How many moles are in 250 grams of tungsten (w)? * 4.4x10^23 moles 4.2x10^23 moles 0.7 moles 1.4 moles
Answers: 3

Chemistry, 22.06.2019 09:40
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3

Chemistry, 22.06.2019 17:30
I'm learning about the periodic tables and what each subject's configuration is. for example, hydrogen is 1s^1, but i don't understand how you get that. can someone me understand how to figure out how to figure this out? sorry if the question makes no sense, but it would really a lot if you could me understand! you so much if you can!
Answers: 1

Chemistry, 23.06.2019 02:30
Which words or phrases identify layers of groundwater? check all that apply. water table kettle lake saturation zone underground lake sinkhole will give brainiest, answer quickly.
Answers: 1
You know the right answer?
You need to calculate the enthalpy change (AH) of the reaction A + B → C.
How can the enthalpies gi...
Questions


Mathematics, 16.12.2021 21:20



Mathematics, 16.12.2021 21:20


Mathematics, 16.12.2021 21:20




Mathematics, 16.12.2021 21:20



Mathematics, 16.12.2021 21:20









