

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:10
Which of the following elements would you expect to have the lowest ionization energy value? fluorine, lithium, neon, nitrogen
Answers: 2

Chemistry, 22.06.2019 11:00
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 20:20
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1

Chemistry, 23.06.2019 02:00
The plant food contains nh4)3po4 what tests would you run to verify the presence of the nh4 ion and the po4 ion
Answers: 2
You know the right answer?
the density of gold is 19.3 g/ml. if you had a cube made of pure gold that weighs 58.1 pounds what w...
Questions

Mathematics, 28.04.2021 15:40


English, 28.04.2021 15:40

Mathematics, 28.04.2021 15:40



Chemistry, 28.04.2021 15:40

English, 28.04.2021 15:40


Mathematics, 28.04.2021 15:40


Physics, 28.04.2021 15:40

Biology, 28.04.2021 15:50



Geography, 28.04.2021 15:50

History, 28.04.2021 15:50

Mathematics, 28.04.2021 15:50

Computers and Technology, 28.04.2021 15:50

Mathematics, 28.04.2021 15:50

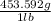 = 26353.69 g
= 26353.69 g

