
Chemistry, 12.06.2021 16:20 laiba012305
A 5.00 mL sample of vinegar, a solution of acetic acid, is titrated with 0.756 M calcium hydroxide. 10.23 mL of the calcium hydroxide is required to reach the equivalence point. What is the molarity of the acetic acid in the vinegar.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 09:30
Which element is the least metallic between cadmium, silver, zinc, or iron?
Answers: 1

Chemistry, 22.06.2019 21:00
Rays from the sun are not considered matter true or false
Answers: 2

Chemistry, 23.06.2019 00:30
When a beta particle is emitted, the mass number of the nucleus a. decreases by one b. increases by one c. remains the same d. decreases by two
Answers: 2

Chemistry, 23.06.2019 01:20
How can parts of a solution be separated by chromatography?
Answers: 1
You know the right answer?
A 5.00 mL sample of vinegar, a solution of acetic acid, is titrated with 0.756 M calcium hydroxide....
Questions

Social Studies, 30.08.2019 16:30

Mathematics, 30.08.2019 16:30

English, 30.08.2019 16:30




English, 30.08.2019 16:30






History, 30.08.2019 16:30

Computers and Technology, 30.08.2019 16:30

History, 30.08.2019 16:30

Physics, 30.08.2019 16:30


Mathematics, 30.08.2019 16:30


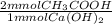 = 15.46 mmol CH₃COOH
= 15.46 mmol CH₃COOH

