
Chemistry, 12.06.2021 19:30 myaaa13754
During an exothermic reaction, H for the reactants was 890 kJ/mol. Which of the following statements is correct about the H for the products and the comparison of the energy in bonds?
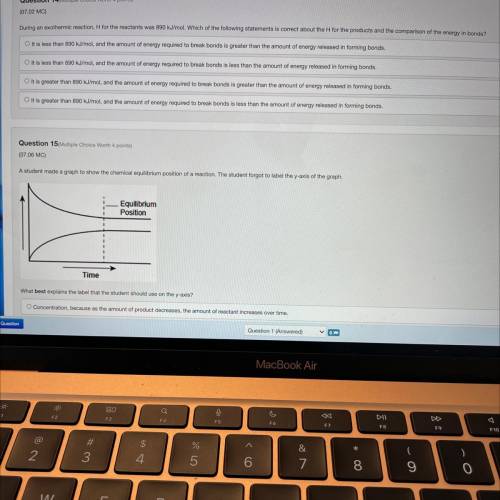

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 1

Chemistry, 22.06.2019 14:10
Precision can be defined as the o exact center of a data set. o reproducibility of a measured value. o correlation between two variables that are measured in a data set agreement between a measured value and an accepted value.
Answers: 2

Chemistry, 22.06.2019 22:30
What methods could you use to solubilize calcium carbonate
Answers: 1

Chemistry, 23.06.2019 01:30
Astudent states that 9.0 g of baking soda will form an unsaturated solution in 100 g of water. what do you need to know to decide whether this statement is correct? a. the temperature of the water and the molar mass of baking soda b. the percent by volume of the solution and the solubility of baking soda c. the temperature of the water and the solubility of baking soda at that temperature
Answers: 1
You know the right answer?
During an exothermic reaction, H for the reactants was 890 kJ/mol. Which of the following statements...
Questions

Mathematics, 02.11.2020 21:40

World Languages, 02.11.2020 21:40

Physics, 02.11.2020 21:40


History, 02.11.2020 21:40

Mathematics, 02.11.2020 21:40




Mathematics, 02.11.2020 21:40

History, 02.11.2020 21:40


English, 02.11.2020 21:40

Mathematics, 02.11.2020 21:40

Mathematics, 02.11.2020 21:40

History, 02.11.2020 21:40

Chemistry, 02.11.2020 21:40

Mathematics, 02.11.2020 21:40


Chemistry, 02.11.2020 21:40



