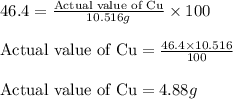Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 1

Chemistry, 22.06.2019 12:30
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1

Chemistry, 22.06.2019 17:00
Astable electron arrangement for an atom is one that does not easily change. how is this arrangement arrived at? a. valence electrons are transferred or shared to create a full outer shell of electrons. b. valence electrons are discarded into space to create a full outer shell of electrons. c. protons (positive charge) pair with valence electrons (negative charge) to create a strong bond. d. outer shells with valence electrons are transferred or shared.
Answers: 2

Chemistry, 22.06.2019 18:10
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1
You know the right answer?
Aluminum reacts with excess copper(II) sulfate according to the unbalanced reaction
Al(s) + CuSO4(a...
Questions











Mathematics, 08.12.2020 08:00

Biology, 08.12.2020 08:00


Mathematics, 08.12.2020 08:00


Mathematics, 08.12.2020 08:00

Mathematics, 08.12.2020 08:00

Social Studies, 08.12.2020 08:00

Mathematics, 08.12.2020 08:00

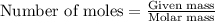 ......(1)
......(1)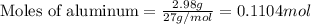
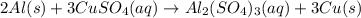
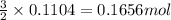 of Cu
of Cu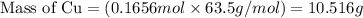
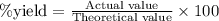 ......(2)
......(2)