
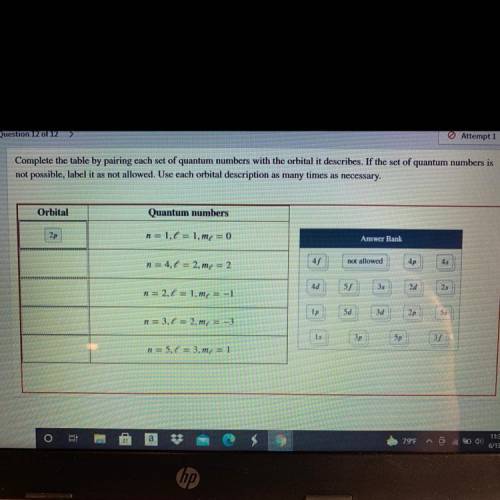
Complete the table by pairing each set of quantum numbers with the orbital it describes. If the set of quantum numbers is
not possible, label it as not allowed. Use each orbital description as many times as necessary.
Orbital
Quantum numbers
2p
n = 1.6 = 1.me = 0
Answer Bank
n = 4.1 = 2.m = 2
45
not allowed
4p
45
4d
n = 2.1 = 1.mx = -1
55
35
2d
25
Ip
5d
3d
2p
55
n = 3,6 = 2.mx = -3
1s
3p
5p
35
n = 5.0 = 3.m4 = 1
7
O
Bi
11:36 PM

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 10:00
The tendency of water molecules to stick together is referred to as a) adhesion b) polarity c) cohesion d) transpiration e) evaporation
Answers: 1

Chemistry, 22.06.2019 16:30
At 20°c, a sample of h2o liquid and a sample of co2 gas each have the same average kinetic energy. why is one a liquid and the other a gas at this temperature?
Answers: 1

Chemistry, 22.06.2019 21:00
Acandle’s wick is the fabric string that holds the flame, and it burns down at a constant slow pace when the candle is lit. the wick is usually surrounded by wax. which is the most important property of covalent compounds that makes them useful for making candle wax? a low boiling point a low melting point a high boiling point a high melting point
Answers: 1

Chemistry, 23.06.2019 07:00
Scuba divers use tanks of compressed air to them breathe. gases can be compressed because?
Answers: 1
You know the right answer?
Complete the table by pairing each set of quantum numbers with the orbital it describes. If the set...
Questions

Biology, 23.06.2019 05:30


Social Studies, 23.06.2019 05:30

Social Studies, 23.06.2019 05:30

History, 23.06.2019 05:30

Advanced Placement (AP), 23.06.2019 05:30

Mathematics, 23.06.2019 05:30

Mathematics, 23.06.2019 05:30

Mathematics, 23.06.2019 05:30



Mathematics, 23.06.2019 05:30


Health, 23.06.2019 05:30


Mathematics, 23.06.2019 05:30

Chemistry, 23.06.2019 05:30

English, 23.06.2019 05:30


Mathematics, 23.06.2019 05:30



