

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:50
Which best describes why nh4+ can form an ionic bond with cl-?
Answers: 3

Chemistry, 22.06.2019 02:00
How many moles of magnesium is 3.01 x10^22 atoms of magnesium?
Answers: 1

Chemistry, 22.06.2019 09:00
The diagram below shows a cell placed in a solution.a cell is shown placed inside a beaker. it is labeled cell. the solution inside the beaker is labeled 40% salt solution and the solution inside the cell is labeled 20% salt solution.only water is allowed to move in and out of the cell. what will most likely happen to the cell? it will expand as water moves out of it. it will shrink as water moves out of it.it will expand as water moves into it. it will shrink as water moves into it.
Answers: 2

Chemistry, 22.06.2019 14:30
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3
You know the right answer?
Which of the following molecules would you expect to have a dipole moment of zero? a, CH2 Ch3
bH2C=...
Questions

Mathematics, 23.10.2020 04:01

Mathematics, 23.10.2020 04:01



Mathematics, 23.10.2020 04:01

Mathematics, 23.10.2020 04:01

Mathematics, 23.10.2020 04:01


Biology, 23.10.2020 04:01



History, 23.10.2020 04:01


Mathematics, 23.10.2020 04:01

Mathematics, 23.10.2020 04:01

Mathematics, 23.10.2020 04:01


Biology, 23.10.2020 04:01


Mathematics, 23.10.2020 04:01

 is expected to have a dipole moment of zero.
is expected to have a dipole moment of zero. distance (in cm)
distance (in cm)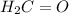 is a polar molecule so it will have dipole moment.
is a polar molecule so it will have dipole moment.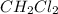 is a polar molecule so it will have dipole moment.
is a polar molecule so it will have dipole moment.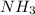 has nitrogen atom as more electronegative than hydrogen atom. So, net dipole moment will be in the direction of nitrogen atom.
has nitrogen atom as more electronegative than hydrogen atom. So, net dipole moment will be in the direction of nitrogen atom.

