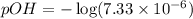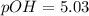Chemistry, 14.06.2021 15:20 galaxychild101
A solution of acetic acid that has a concentration of 0.10 moles per liter has a pH of 2.87. What is the likely pH of a 0.10 mole per liter solution of the conjugate base sodium acetate?
A. 8.97
B. 1.00
C. 2.87
D. 4.74
E. 13.00

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 09:00
Identify the electromagnets with poles that are reversed from the electromagnet shown above
Answers: 3


Chemistry, 22.06.2019 16:40
The diagram below shows the movement of particles. what does this piece of evidence best support? the collision theory the maxwell-boltzmann distribution the effect of pressure on reaction rates the effect of temperature on reaction rates
Answers: 3

Chemistry, 22.06.2019 17:30
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2
You know the right answer?
A solution of acetic acid that has a concentration of 0.10 moles per liter has a pH of 2.87. What is...
Questions




Health, 04.11.2020 22:50

Mathematics, 04.11.2020 22:50


English, 04.11.2020 22:50

Chemistry, 04.11.2020 22:50






Mathematics, 04.11.2020 22:50

Social Studies, 04.11.2020 22:50



Mathematics, 04.11.2020 22:50

Mathematics, 04.11.2020 22:50

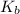 of a reaction, we use the equation:
of a reaction, we use the equation: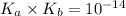
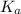 = acid dissociation constant of acetic acid =
= acid dissociation constant of acetic acid = 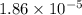
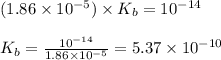
![[OH^-]=\sqrt{K_b\times \text{[Conjugate base]}}](/tpl/images/1373/6739/be895.png)
![[OH^-]=\sqrt{(5.37\times 10^{-10})\times 0.1}](/tpl/images/1373/6739/6c6fd.png)
![[OH^-]=7.33\times 10^{-6}](/tpl/images/1373/6739/66957.png)
![pOH=-\log [OH^-]](/tpl/images/1373/6739/1fac1.png)