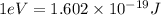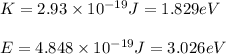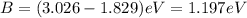(A) Calculate the wavelength (in nm) of light with energy 1.89 × 10–20 J per photon, (b) For light of wavelength 410 nm, calculate the number of photons per joule, (c) Determine the binding energy (in eV) of a metal if the kinetic energy possessed by an ejected electron [using one of the photons in part (b)] is 2.93 × 10–19 J.

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 19:30
Helium decays to form lithium. which equation correctly describes this decay?
Answers: 2

Chemistry, 23.06.2019 14:20
Identificaa 5 características que comparten las guacamayas y dos caracteristicas que las hagan diferentes
Answers: 1

Chemistry, 23.06.2019 16:00
What is the consequence on your brain for snorting or taking amphetamines(meth/stimulant) as a 16 year old(minor)?
Answers: 2
You know the right answer?
(A) Calculate the wavelength (in nm) of light with energy 1.89 × 10–20 J per photon, (b) For light o...
Questions

History, 09.01.2021 01:10

Mathematics, 09.01.2021 01:10


Mathematics, 09.01.2021 01:10

Mathematics, 09.01.2021 01:10



Social Studies, 09.01.2021 01:10

English, 09.01.2021 01:10



English, 09.01.2021 01:10

History, 09.01.2021 01:10




Mathematics, 09.01.2021 01:10



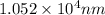
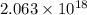
 ......(1)
......(1)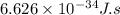
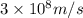
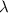 = wavelength
= wavelength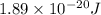
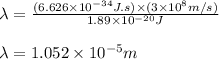
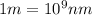
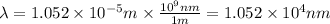
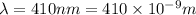
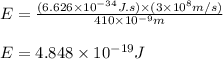
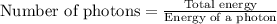
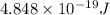
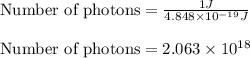
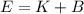 .....(2)
.....(2)