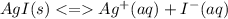Chemistry, 14.06.2021 21:00 jungkookie001
Compare the solubility of silver iodide in each of the following aqueous solutions:
a. 0.10 M AgCH3COO
b. 0.10 M NaI
c. 0.10 M KCH3COO
d. 0.10 M NH4NO3
1. More soluble than in pure water.
2. Similar solubility as in pure water.
3. Less soluble than in pure water.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:30
1.aluminum chloride (alcl3), and sodium hydroxide (naoh) can react to form aluminum hydroxide (al(oh)3) and sodium chloride (nacl). you have 13.4 g of aluminum chloride and 10.0 g of sodium hydroxide. answer the following questions: •what is the balanced equation for this reaction? •if you use all 13.4 g of aluminum chloride, how many grams of aluminum hydroxide can be formed? work must be shown to earn credit •if you use all 10.0 g of sodium hydroxide, how many grams of aluminum hydroxide can be formed? work must be shown to earn credit •how many grams of aluminum hydroxide will actually be made? which reagent is limiting? explain your answer.
Answers: 1

Chemistry, 22.06.2019 01:00
Which of the following is always a reactant in a combustion reaction? oxygen nitrogen hydrogen carbon
Answers: 1

Chemistry, 22.06.2019 04:30
Use the periodic table to determine the electron configuration of dysprosium (dy) and americium (am) in noble-gas notation.
Answers: 1

Chemistry, 22.06.2019 06:30
What effect might melting sea ice have for people who live in coastal areas?
Answers: 1
You know the right answer?
Compare the solubility of silver iodide in each of the following aqueous solutions:
a. 0.10 M AgCH3...
Questions

Mathematics, 04.11.2020 23:40

Chemistry, 04.11.2020 23:40

Mathematics, 04.11.2020 23:40


Mathematics, 04.11.2020 23:40

Mathematics, 04.11.2020 23:40

Social Studies, 04.11.2020 23:40


Mathematics, 04.11.2020 23:40





Arts, 04.11.2020 23:40

Mathematics, 04.11.2020 23:40


Mathematics, 04.11.2020 23:40

Health, 04.11.2020 23:40

Mathematics, 04.11.2020 23:40

Mathematics, 04.11.2020 23:40