Select
Dictionary
Guided Practice
After reviewing the above information and the videos...

Chemistry, 14.06.2021 23:40 briarkaltvedt
Select
Dictionary
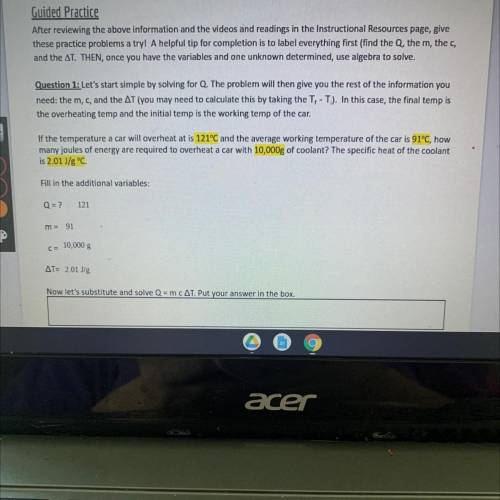
Guided Practice
After reviewing the above information and the videos and readings in the Instructional Resources page, give
these practice problems a tryl A helpful tip for completion is to label everything first (find the Q, the m, the c,
and the AT. THEN, once you have the variables and one unknown determined, use algebra to solve.
Text to Speech
Markup
Question 1: Let's start simple by solving Q. The problem will then give you the rest of the information you
need: the m, c, and the AT (you may need to calculate this by taking the T, -T). In this case, the final temp is
the overheating temp and the initial temp is the working temp of the car.
Comment
Text Box
14 -
If the temperature a car will overheat at is 121°C and the average working temperature of the car is 91°C, how
many joules of energy are required to overheat a car with 10,000g of coolant? The specific heat of the coolant
is 2.01 J/g °C.
Equation
Fill in the additional variables:
Drawing
Q = ?
121
Shapes
m = 91
Eraser
C= 10,000 g
Add Media
AT= 2.01 Jg
Signature
Now let's substitute and solve Q = m CAT. Put your answer in the box.
<

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 13:30
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1


Chemistry, 22.06.2019 23:10
Using the periodic table, complete the following. element: hydrogen symbol: h₂ molecular weight: g mass of one mole: g/mol
Answers: 3
You know the right answer?
Questions

History, 27.01.2020 20:31


Mathematics, 27.01.2020 20:31




Social Studies, 27.01.2020 20:31

Mathematics, 27.01.2020 20:31

Biology, 27.01.2020 20:31

Mathematics, 27.01.2020 20:31



Biology, 27.01.2020 20:31


Mathematics, 27.01.2020 20:31

Chemistry, 27.01.2020 20:31


History, 27.01.2020 20:31

Physics, 27.01.2020 20:31



