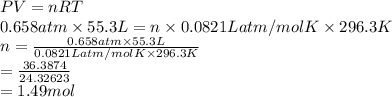Chemistry, 15.06.2021 06:30 aidanfbussiness
A sample of gas occupies a volume of 55.3 L, has a temperature of 23.3 °C and a pressure of .658 atm. Calculate the number of moles of gas which are present in the sample. R= .0821 atm L/mol K

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 14:00
Classify each statement about effective nuclear charge, zeff, as true or false.
Answers: 2

Chemistry, 22.06.2019 05:30
What royal scientist used the 29th day of frozen vapor to encounter elements for mastering new culinary creations?
Answers: 1

Chemistry, 22.06.2019 07:00
If there is any 12 to 14 girls that need a boyfriend just follow me and let me know
Answers: 1

Chemistry, 22.06.2019 09:00
Chemical energy is a form of a. kinetic energy only. b. both potential and kinetic energy. c. neither potential nor kinetic energy. d. potential energy only. reset
Answers: 1
You know the right answer?
A sample of gas occupies a volume of 55.3 L, has a temperature of 23.3 °C and a pressure of .658 atm...
Questions

Mathematics, 09.03.2021 18:50


Spanish, 09.03.2021 18:50

Biology, 09.03.2021 18:50




Mathematics, 09.03.2021 18:50

Mathematics, 09.03.2021 18:50

Mathematics, 09.03.2021 18:50




Mathematics, 09.03.2021 18:50

Mathematics, 09.03.2021 18:50



Computers and Technology, 09.03.2021 18:50

Biology, 09.03.2021 18:50

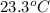 = (23.3 + 273) K = 296.3 K
= (23.3 + 273) K = 296.3 K