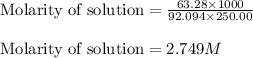Chemistry, 15.06.2021 14:00 msladycie8831
Glycerol. C3HgO3, is a substance used extensively in the manufacture of cosmetics, foodstuffs, antifreeze, and plastics. Glycerol is a water-soluble liquid
with a density of 1.2656 g/mL at 15 °C. Calculate the molarity of a solution of glycerol made by dissolving 50.000 mL glycerol at 15 °C in enough water to
make 250.00 mL of solution. The molecular weight of C3HgO3 is 92.094 amu.
O A 0.6871
O B. 3.600
O C. 63.28
O 0.92.10
O E. 2.749

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:30
List the two type of transporst that the cell in orde to transport molecules acroos the membrane
Answers: 1

Chemistry, 22.06.2019 02:30
Based on the equation and the information in the table, what is the enthalpy of the reaction?
Answers: 2

Chemistry, 22.06.2019 05:30
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 09:00
Look at the spectrums of a star moving towards earth and a motionless star. which of these is a correct inference that can be draw from the observation of the two spectrums? (2 points) the spectrum of a motionless star is difficult to be viewed separately using oridinary telescopes. the spectrum of a motionless star is identical to the spectrum of a star which moves towards earth. the spectrum of a star shifts towards the red region when the star moves towards earth. the spectrum of a star shifts towards the blue region when the star moves towards earth.
Answers: 2
You know the right answer?
Glycerol. C3HgO3, is a substance used extensively in the manufacture of cosmetics, foodstuffs, antif...
Questions



Mathematics, 18.10.2019 07:30

Mathematics, 18.10.2019 07:30

Arts, 18.10.2019 07:30






Mathematics, 18.10.2019 07:30




Geography, 18.10.2019 07:30

History, 18.10.2019 07:30




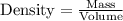 ......(1)
......(1)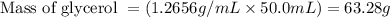
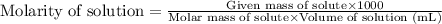 .....(2)
.....(2)