
Chemistry, 15.06.2021 18:20 aliciabenitez
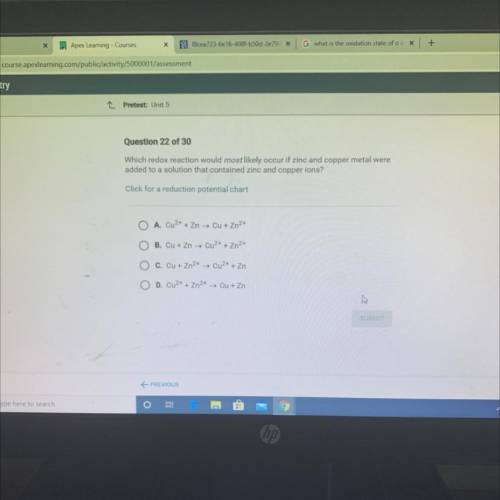
Which redox reaction would most likely occur if zinc and copper metal were
added to a solution that contained zinc and copper ions?
Click for a reduction potential chart
A. Cu2+ + Zn → Cu + Zn2+
O B. Cu + Zn → Cu2+ + Zn2+
C. Cu + Zn2+ → Cu2+ + Zn
O D. Cu2+ + Zn2+ → Cu + Zn

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 21:00
Write the chemical symbols for three different atoms or atomic cations with 27 electrons. asap!
Answers: 2

Chemistry, 22.06.2019 02:50
What is the overall order of reaction for rate = k[no]2[o2]
Answers: 3

Chemistry, 22.06.2019 06:30
What is the correct term for living the most sustainable life you can within your current circumstances?
Answers: 1

Chemistry, 22.06.2019 14:30
Ahypothesis must be testable and falsifiable to be considered scientific a. trueb. false
Answers: 1
You know the right answer?
Which redox reaction would most likely occur if zinc and copper metal were
added to a solution that...
Questions

Mathematics, 02.11.2019 01:31

Mathematics, 02.11.2019 01:31


Mathematics, 02.11.2019 01:31

History, 02.11.2019 01:31






Mathematics, 02.11.2019 01:31

Social Studies, 02.11.2019 01:31

Mathematics, 02.11.2019 01:31

Computers and Technology, 02.11.2019 01:31



English, 02.11.2019 01:31

History, 02.11.2019 01:31


History, 02.11.2019 01:31



