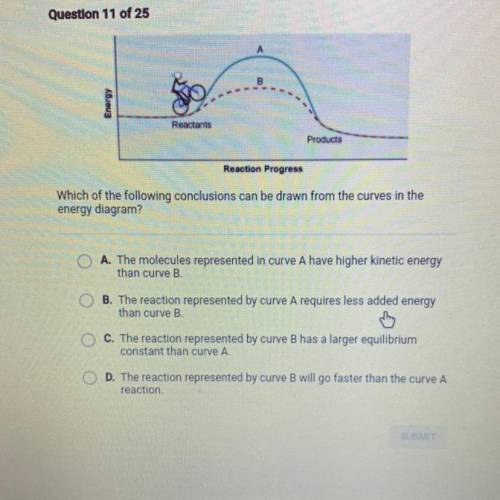
Question 11 of 25
Reactants
Products
Reaction Progress
Which of the following con...

Chemistry, 15.06.2021 19:00 lilpeepxliltracy
Question 11 of 25
Reactants
Products
Reaction Progress
Which of the following conclusions can be drawn from the curves in the
energy diagram?
O A. The molecules represented in curve A have higher kinetic energy
than curve B.
OB. The reaction represented by curve A requires less added energy
than curve B.
dh
C. The reaction represented by curve B has a larger equilibrium
constant than curve A.
D. The reaction represented by curve B will go faster than the curve A
reaction

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:30
Aspirin has a density of 1.40 g/cm3 what is the volume in cubic centimeters of a tablet weighing 320 mg ?
Answers: 1

Chemistry, 21.06.2019 19:30
The molecular formula for caffeine is cshion402. which of the following elements is not found in caffeine?
Answers: 1

Chemistry, 22.06.2019 02:30
When you perform this reaction, what could remain at the end of the reaction? check all that apply. excess reactant aqueous copper chloride excess reactant aluminum oxygen product solid copper carbon dioxide product aqueous aluminum chloride water
Answers: 2

Chemistry, 22.06.2019 09:00
Given the following reaction: c3h8+5o2=3co2+4h20 how many grams of co2 will be produced 7 g of c3h8 and 98 g of o2
Answers: 1
You know the right answer?
Questions



Mathematics, 30.03.2020 19:00

Chemistry, 30.03.2020 19:00

Mathematics, 30.03.2020 19:00





Mathematics, 30.03.2020 19:00

Computers and Technology, 30.03.2020 19:00











