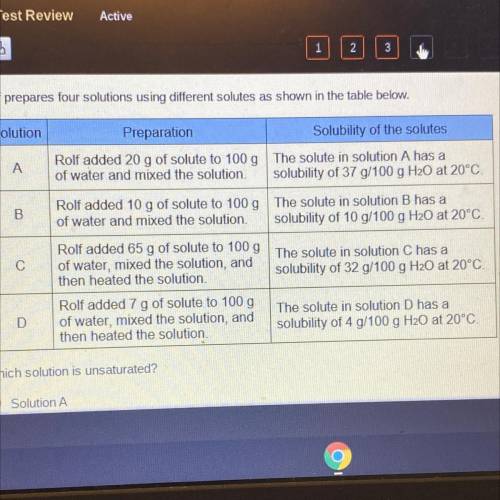
Rolf prepares four solutions using different solutes as shown in the table below.
Solution
Pr...

Rolf prepares four solutions using different solutes as shown in the table below.
Solution
Preparation
Solubility of the solutes
A
Rolf added 20 g of solute to 100 g The solute in solution A has a
of water and mixed the solution solubility of 37 g/100 g H20 at 20°C!
B
Rolf added 10 g of solute to 100 g The solute in solution B has a
of water and mixed the solution. solubility of 10 g/100 g H20 at 20°C.
С
Rolf added 65 g of solute to 100g The solute in solution C has a
of water, mixed the solution, and
then heated the solution,
solubility of 32 g/100 g H20 at 20°C
D
Rolf added 7 g of solute to 100 g
of water, mixed the solution, and
then heated the solution
The solute in solution D has a
solubility of 4 g/100 g H20 at 20°C.
Which solution is unsaturated?

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 16:50
Which element is least likely to undergo a chemical reaction
Answers: 3

Chemistry, 23.06.2019 01:00
What two factors can affect the properties of a hydrocarbon? a. the number of its carbon atoms and the number of single bonds b. the number of its carbon atoms and the arrangement of its atoms c. the arrangement of its atoms and the number of its double bonds
Answers: 1

Chemistry, 23.06.2019 03:30
Which of the following describes the entropy change as a solution is made from a liquid and solid
Answers: 1
You know the right answer?
Questions


Computers and Technology, 03.01.2020 04:31

Social Studies, 03.01.2020 04:31




Physics, 03.01.2020 04:31





Mathematics, 03.01.2020 04:31

Mathematics, 03.01.2020 04:31





Mathematics, 03.01.2020 04:31


History, 03.01.2020 04:31



