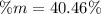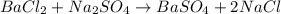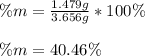Chemistry, 16.06.2021 01:00 chodister353
A mixture of BaCl2 and NaCl is analyzed by precipitating all the barium as BaSO4. After addition of an excess of Na2SO4 to a 3.656-g sample of the mixture, the mass of precipitate collected is 1.658 g. What is the mass percentage of barium chloride in the mixture

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which type of bond is present in hydrogen sulfide (h2s)? the table of electronegativities is given. a. hydrogen b. ionic c. nonpolar covalent d. polar covalent
Answers: 1

Chemistry, 21.06.2019 22:40
How many electrons does silver have to give up in order to achieve a sido noble gas electron configuration
Answers: 1

Chemistry, 22.06.2019 02:20
6. what does the symbol ah stand for? o one calorie given off by a reaction the specific heat of a substance the heat capacity of a substance the heat of reaction for a chemical reaction
Answers: 1

Chemistry, 22.06.2019 04:30
Using the periodic table, complete the table to describe each atom. type in your answers
Answers: 3
You know the right answer?
A mixture of BaCl2 and NaCl is analyzed by precipitating all the barium as BaSO4. After addition of...
Questions








Mathematics, 12.01.2020 18:31

Mathematics, 12.01.2020 18:31

History, 12.01.2020 18:31


Chemistry, 12.01.2020 18:31



Computers and Technology, 12.01.2020 18:31

History, 12.01.2020 18:31

Health, 12.01.2020 18:31

Mathematics, 12.01.2020 18:31


Mathematics, 12.01.2020 18:31