
Chemistry, 16.06.2021 07:30 babygirl123468
An inflated balloon contains X air molecules. After some time the volume of the balloon is found to be the half at the same temperature and pressure when a few air molecules are expelled out. a)How many molecules will be there in the balloon now? b) Which is the gas law associated with this?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:00
An electrons position cannot be known precisely only it's probability of being in a certain location can be known
Answers: 1

Chemistry, 22.06.2019 10:30
When the speed of the bottle is 2 m/s, the average maximum height of the beanbag is m.
Answers: 2

Chemistry, 22.06.2019 11:00
Ais a mountain created from eruptions of lava, ash, rocks, and hot gases.
Answers: 1

Chemistry, 22.06.2019 11:00
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1
You know the right answer?
An inflated balloon contains X air molecules. After some time the volume of the balloon is found to...
Questions





Biology, 18.11.2020 16:40

Biology, 18.11.2020 16:40


Mathematics, 18.11.2020 16:40





Mathematics, 18.11.2020 16:40

Biology, 18.11.2020 16:40

Mathematics, 18.11.2020 16:40

History, 18.11.2020 16:40

English, 18.11.2020 16:40



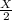 number of molecules now.
number of molecules now. (At constant pressure and temperature)
(At constant pressure and temperature)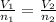 .....(1)
.....(1) = Initial volume and number of moles
= Initial volume and number of moles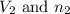 = Final volume and number of moles
= Final volume and number of moles

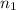 number of moles of gas contains X number of molecules
number of moles of gas contains X number of molecules number of moles of gas will contain =
number of moles of gas will contain =  number of molecules
number of molecules

