
Chemistry, 11.10.2019 19:30 rowdycar313p0ao5k
What is the theoretical yield of ammonia that can be obtained from the reaction of 10.0 g of h2 and excess n2?
n2 + 3h2 → 2nh3
a. 90.0 g
b. 97.1 g
c. 56.3 g
d. 48.6 g
e. 28.4 g

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1


Chemistry, 23.06.2019 11:00
Intermolecular forces. question i need with: the only intermolecular forces that affect non polar molecules are forces.
Answers: 2

Chemistry, 23.06.2019 14:50
Select the correct answer from each drop-down menu. in the process of nuclear fission, . fission only happens to very atoms. the fission process usually also produces several free .
Answers: 2
You know the right answer?
What is the theoretical yield of ammonia that can be obtained from the reaction of 10.0 g of h2 and...
Questions

Mathematics, 26.05.2020 17:58



Arts, 26.05.2020 17:58



Mathematics, 26.05.2020 17:58




English, 26.05.2020 17:58

Mathematics, 26.05.2020 17:58

Mathematics, 26.05.2020 17:58



Biology, 26.05.2020 17:58

History, 26.05.2020 17:58


Mathematics, 26.05.2020 17:58

Physics, 26.05.2020 17:58

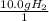 ×
×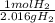 ×
×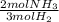 ×
×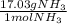 =56.3gNH↓3
=56.3gNH↓3

