
Need help ASAP, please only answer if your willing to help, not for points, and I’ll give brainiest
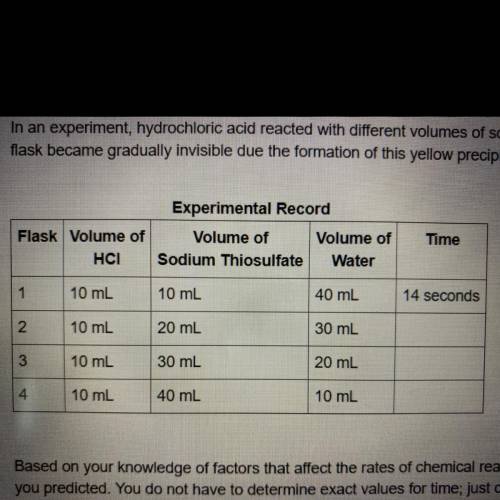
In an experiment, hydrochloric acid reacted with different volumes of sodium thiosulfate in water. A yellow precipitate was formed during the reaction. A cross drawn at the base of each
flask became gradually invisible due the formation of this yellow precipitate. The time taken for the cross to become invisible was recorded. A partial record of the experiment is shown.
Based on your knowledge of factors that affect the rates chemical reactions, predict the trend in the last column of the experimental record. Use complete sentences to explain the trend
you predicted. You do not have to determine exact values for time; just describe the trend you would expect (increase or decrease) and why it occurs.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 13:30
Astudent is trying to create a table that compares hypotheses, theories, and laws. hypothesis theory law do scientific researchers formulate it? yes yes yes does it explain why things happen? yes yes no yes yes yes is it used to make predictions? no yes yes which of the following questions would most likely fill the blank in the table? is it an intelligent guess? is it newly formulated? is it based on observations? has it been proved?
Answers: 1

Chemistry, 22.06.2019 20:30
What is a difference between a mixture of elements and a mixture of compounds
Answers: 1


Chemistry, 23.06.2019 13:00
What type of reaction is this equation c2h5s + o2 > co2 + h2o + so2
Answers: 2
You know the right answer?
Need help ASAP, please only answer if your willing to help, not for points, and I’ll give brainiest...
Questions


Mathematics, 21.03.2020 04:19













Health, 21.03.2020 04:20



English, 21.03.2020 04:20




