
Chemistry, 16.06.2021 22:20 erica11223344
Apply the law of mass action to determine the equilibrium expression for
2NO2Cl(aq) =2NO2 (aq) + Cl2 (aq)
OA. K=[NO_ Cl]?[NO2]?[ciz]
[NO_C1]
[NO2]?[Ciz]
OB, K=
Ock=
2[NO_IC12]
2[NO2C]
OD. K=
2[NO2Cl]
2[NO2][Ciz]
OEK=
[NO2][C12]
[NO_C1]?
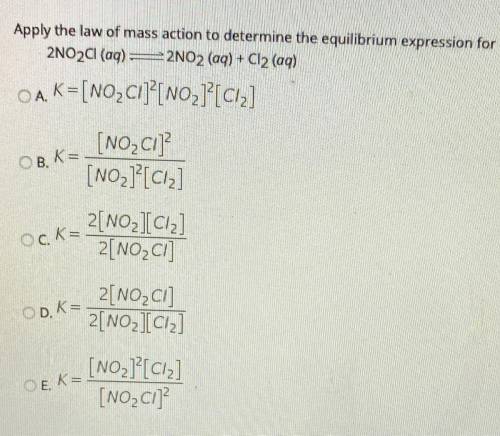

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 22:30
What is the work done by the electric force to move a 1 c charge from a to b?
Answers: 2

Chemistry, 23.06.2019 06:30
Acompound has the molecular formula c3h8. which class of organic compounds does it belong to?
Answers: 1

Chemistry, 23.06.2019 13:30
What would happen if no were added to n(g)+o2=2no(g) at equilibrium?
Answers: 1
You know the right answer?
Apply the law of mass action to determine the equilibrium expression for
2NO2Cl(aq) =2NO2 (aq) + Cl...
Questions



Biology, 18.03.2021 20:40




English, 18.03.2021 20:40

Mathematics, 18.03.2021 20:40


English, 18.03.2021 20:40

English, 18.03.2021 20:40




Mathematics, 18.03.2021 20:40

Law, 18.03.2021 20:40


Mathematics, 18.03.2021 20:40

History, 18.03.2021 20:40



