.
Given the equation
2A(g) - 2B(g) + C(g)
the equilibrium constant is 0.0112 at 140°C....

Chemistry, 16.06.2021 22:20 emilyphillips1681
.
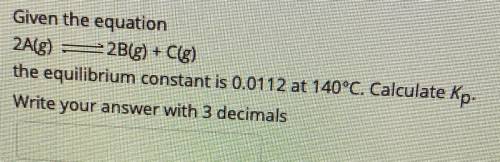
Given the equation
2A(g) - 2B(g) + C(g)
the equilibrium constant is 0.0112 at 140°C. Calculate Kp.
Write your answer with 3 decimals

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:30
The big bang nucleosynthesis theory states that elements were produced in the first few minutes of the big bang while elements have their origins in the interiors of stars, forming much later in the history of the universe.
Answers: 1

Chemistry, 22.06.2019 20:30
Which states of matter have particles that move independently of one another with very little attraction?
Answers: 1

Chemistry, 22.06.2019 21:30
Which of the following changes will decrease the total amount of gaseous solute able to be dissolved in a liter of liquid water? (2 points) decreasing temperature decreasing pressure decreasing surface area decreasing solute concentration
Answers: 1

You know the right answer?
Questions


Biology, 03.05.2021 17:50

Mathematics, 03.05.2021 17:50

Mathematics, 03.05.2021 17:50


Social Studies, 03.05.2021 17:50




Spanish, 03.05.2021 17:50



English, 03.05.2021 17:50




Physics, 03.05.2021 17:50





