

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:00
Select the correct answer. which statement is true about a polarized object? o a. it gains electrons and becomes negatively charged. ob. it gains protons and becomes positively charged. oc. the number of positive and negative charges can be the same. od. it has to be a metal. o e. there is no change in the distribution of the charge in the object. reset next what
Answers: 3


Chemistry, 22.06.2019 20:00
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2

Chemistry, 23.06.2019 03:30
If you need to add 27.50ml of a solution, which piece of glassware would you use to deliver this volume and explain how you would determine if the 27.50 ml was measured?
Answers: 2
You know the right answer?
In a 0.730 M solution, a weak acid is 12.5% dissociated. Calculate Ka of the acid....
Questions



English, 09.09.2021 04:20

Chemistry, 09.09.2021 04:20

Mathematics, 09.09.2021 04:20



Mathematics, 09.09.2021 04:20

English, 09.09.2021 04:20

Biology, 09.09.2021 04:20


Mathematics, 09.09.2021 04:20



Mathematics, 09.09.2021 04:20

Social Studies, 09.09.2021 04:20


Mathematics, 09.09.2021 04:20

Mathematics, 09.09.2021 04:20

English, 09.09.2021 04:20

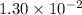 , assuming that this acid is monoprotic.
, assuming that this acid is monoprotic.  denote this acid.
denote this acid.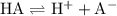 .
.![[{\rm HA}] = 0.730\; \rm mol \cdot L^{-1}](/tpl/images/1377/5071/8d084.png) .
.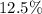 of that was dissociated, the concentration of both
of that was dissociated, the concentration of both 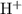 and
and 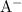 (conjugate base of this acid) would become:
(conjugate base of this acid) would become: 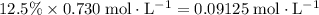 .
. 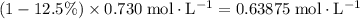 .
.![[{\rm HA}]](/tpl/images/1377/5071/3042a.png) ,
, ![[{\rm H}^{+}]](/tpl/images/1377/5071/23c55.png) , and
, and ![[{\rm A}^{-}]](/tpl/images/1377/5071/9f958.png) denote the concentration (in
denote the concentration (in  or
or 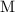 ) of the corresponding species at equilibrium. Calculate the acid dissociation constant
) of the corresponding species at equilibrium. Calculate the acid dissociation constant 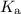 for
for ![\begin{aligned}K_{\rm a} &= \frac{[{\rm H}^{+}] \cdot [{\rm A}^{-}]}{[{\rm HA}]} \\ &= \frac{(0.09125\; \rm mol \cdot L^{-1}) \times (0.09125\; \rm mol \cdot L^{-1})}{0.63875\; \rm mol \cdot L^{-1}}\\[0.5em]&\approx 1.30 \times 10^{-2} \end{aligned}](/tpl/images/1377/5071/efa8b.png) .
.

