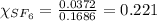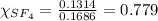Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 15:30
1. baking powder is a 1: 1 molar mixture of cream of tartar (khc4h4o6) and baking soda (nahco3). a recipe calls for two teaspoons (a total of 8.0 grams) of cream of tartar. how much baking soda must be added for both materials to react completely?
Answers: 2

Chemistry, 22.06.2019 07:30
All cells are made of four types of acids, lipids, proteins, and carbohydrates.
Answers: 1

Chemistry, 22.06.2019 09:30
Melissa is interested in her family tree and how her family has changed over its many generations. melissa probably more closely resembles
Answers: 2

Chemistry, 22.06.2019 14:30
Select all of the statements which are true. electrons are located in shells or orbits around the atom. electrons orbit slowly around the atom. electrons travel in one flat path around the nucleus of an atom. the valence of an atom is determined by the number of electrons in the atom's outermost shell.
Answers: 1
You know the right answer?
A tank at is filled with of sulfur tetrafluoride gas and of sulfur hexafluoride gas. You can assume...
Questions



English, 06.02.2021 01:40

Mathematics, 06.02.2021 01:40



Mathematics, 06.02.2021 01:40

Arts, 06.02.2021 01:40


Spanish, 06.02.2021 01:40


Mathematics, 06.02.2021 01:40


Mathematics, 06.02.2021 01:40

Arts, 06.02.2021 01:40

Advanced Placement (AP), 06.02.2021 01:40


Mathematics, 06.02.2021 01:40

Mathematics, 06.02.2021 01:40

Biology, 06.02.2021 01:40

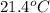 is filled with 5.43 g of sulfur hexafluoride gas and 14.2 g of sulfur tetrafluoride gas. You can assume both gases behave as ideal gases under these conditions. Calculate the mole fraction and partial pressure of each gas. Round each of your answers to significant digits.
is filled with 5.43 g of sulfur hexafluoride gas and 14.2 g of sulfur tetrafluoride gas. You can assume both gases behave as ideal gases under these conditions. Calculate the mole fraction and partial pressure of each gas. Round each of your answers to significant digits. ......(1)
......(1)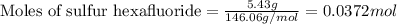
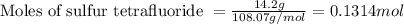
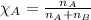 .....(2)
.....(2)