Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:30
Identify the missing numbers below to show the result of multiplying the numbers (1.6 × 10-19)(5.0 × 106) = c × 10d
Answers: 1


Chemistry, 22.06.2019 13:30
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1

Chemistry, 22.06.2019 17:00
The biosphere of the earth is made up of what compound? organic or inorganic?
Answers: 3
You know the right answer?
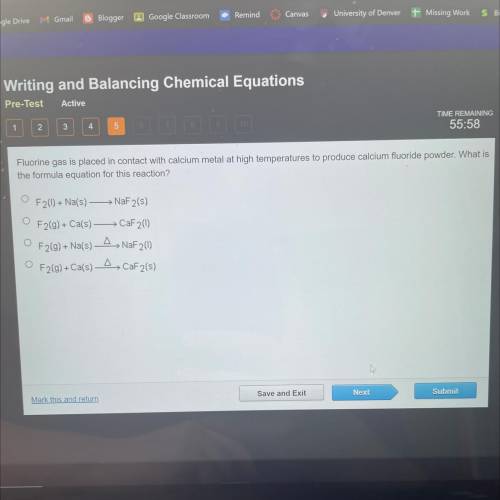
Fluorine gas is placed in contact with calcium metal at high temperatures to produce calcium fluorid...
Questions


Spanish, 17.07.2019 17:50

Biology, 17.07.2019 17:50


Mathematics, 17.07.2019 17:50


Social Studies, 17.07.2019 17:50



Mathematics, 17.07.2019 17:50


English, 17.07.2019 17:50




Spanish, 17.07.2019 17:50

History, 17.07.2019 17:50


English, 17.07.2019 17:50

History, 17.07.2019 17:50