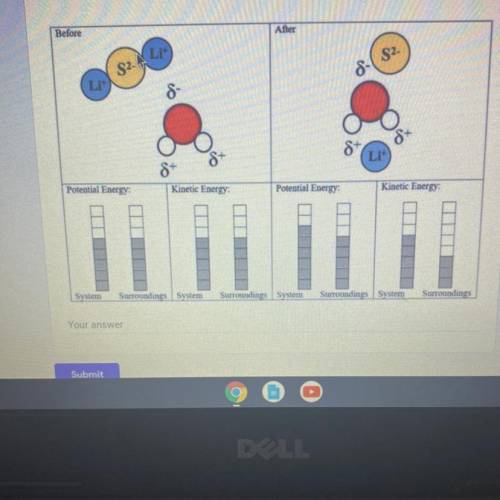
Explain how to fix three errors made by the student who drew this endothermic process model
...

Chemistry, 17.06.2021 22:30 2019jonathanbradford
Explain how to fix three errors made by the student who drew this endothermic process model

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:40
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3

Chemistry, 22.06.2019 06:00
An alkaline battery produces electrical energy according to the following equation. zn(s) + 2 mno2(s) + h2o(l) zn(oh)2(s) + mn2o3(s) (a) determine the limiting reactant if 17.5 g zn and 31.0 g mno2 are used. (type your answer using the format ch4 for ch4.) (b) determine the mass of zn(oh)2 produced. _ g
Answers: 3

Chemistry, 22.06.2019 09:00
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1

Chemistry, 22.06.2019 12:30
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1
You know the right answer?
Questions

Mathematics, 24.07.2020 07:01

Mathematics, 24.07.2020 07:01

Mathematics, 24.07.2020 07:01


English, 24.07.2020 07:01

Mathematics, 24.07.2020 08:01






Mathematics, 24.07.2020 08:01



Mathematics, 24.07.2020 08:01

Chemistry, 24.07.2020 08:01


Mathematics, 24.07.2020 08:01


Spanish, 24.07.2020 08:01



