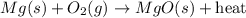Chemistry, 18.06.2021 07:40 adamkinney6110
When heated, magnesium combines readily with excess oxygen in the air to produce magnesium oxide, as shown in the following unbalanced equation.
Mg (s) + O2 (g) → MgO (s) + heat
What two types of reactions could this chemical equation be classified as?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 08:30
The mass of a neutron is equal to the mass of a proton plus the mass of an electron. true or false false true
Answers: 1

Chemistry, 22.06.2019 20:30
Select all the correct answers.which compounds have the empirical formula ch20? (multiple answers)a.c2h4o2b.c3h603c.ch2o2d.c5h1005e.c6h1206
Answers: 2

Chemistry, 23.06.2019 08:00
Technician a says that you should never jump-start a frozen battery. technician b says that a frozen battery can explode, causing injury, when jump-started. who is correct?
Answers: 2

Chemistry, 23.06.2019 11:00
The standard emf for the cell using the overall cell reaction below is +2.20 v: 2al(s) + 3i2(s) → 2ai3+(aq) + 6i-(aq) the emf generated by the cell when [ai3+] = 3.5 × 10-3 m and [i-] = 0.015 m is v. the standard emf for the cell using the overall cell reaction below is +2.20 v: 2al(s) + 3i2(s) 2ai3+(aq) + 6i-(aq) the emf generated by the cell when [ai3+] = 3.5 × 10-3 m and [i-] = 0.015 m is v. 2.36 2.24 2.21 2.51 2.04
Answers: 2
You know the right answer?
When heated, magnesium combines readily with excess oxygen in the air to produce magnesium oxide, as...
Questions



Mathematics, 09.10.2021 04:00

Geography, 09.10.2021 04:00


Social Studies, 09.10.2021 04:00




Mathematics, 09.10.2021 04:00

Social Studies, 09.10.2021 04:00


Mathematics, 09.10.2021 04:00


Mathematics, 09.10.2021 04:00

English, 09.10.2021 04:00

Mathematics, 09.10.2021 04:00

Biology, 09.10.2021 04:00

Mathematics, 09.10.2021 04:00