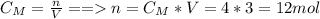You have 4 litres of a 3.0 mol/L solution of NaCl in a
chemical store room.
How many moles of...

Chemistry, 18.06.2021 17:40 donaji1024perez
You have 4 litres of a 3.0 mol/L solution of NaCl in a
chemical store room.
How many moles of NaCl are present? *
0.75 mol
1.33 mol
12 mol

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:30
In a reaction (at equilibrium) that makes more moles of gas than it consumes, what is the effect of increasing the pressure?
Answers: 1



Chemistry, 23.06.2019 06:00
Complete the sentences to best explain the ranking.match the words below to the appropriate blanks in the sentences.a less polar bondhigher molar massion-dipole forcesstronger intermolecular forcesdipole-dipole forcesdispersion forceshydrogen bonding1. h2s and h2se exhibit the following intermolecular forces:.2. therefore, when comparing h2s and h2se the one with a has a higher boiling point .3. the strongest intermolecular force exhibited by h2o is . therefore, when comparing h2se and h2o the one with has a higher boiling point.
Answers: 1
You know the right answer?
Questions

English, 07.10.2020 14:01


Mathematics, 07.10.2020 14:01

Mathematics, 07.10.2020 14:01

History, 07.10.2020 14:01

Mathematics, 07.10.2020 14:01


Social Studies, 07.10.2020 14:01

History, 07.10.2020 14:01

Mathematics, 07.10.2020 14:01

Mathematics, 07.10.2020 14:01



English, 07.10.2020 14:01


Advanced Placement (AP), 07.10.2020 14:01


English, 07.10.2020 14:01