
Chemistry, 18.06.2021 20:00 kassandrarosario1115
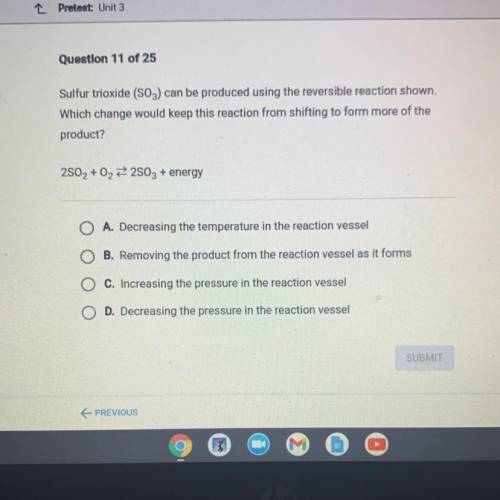
Sulfur trioxide (SO3) can be produced using the reversible reaction shown.
Which change would keep this reaction from shifting to form more of the
product?
2802 + 02 22803 + energy
A. Decreasing the temperature in the reaction vessel
B. Removing the product from the reaction vessel as it forms
C. Increasing the pressure in the reaction vessel
D. Decreasing the pressure in the reaction vessel

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:10
Think about how you can use le chatelier’s principle to find possible solutions to the design problem. describe at least two ways to increase the yield (amount) of ammonia based on this principle.
Answers: 2

Chemistry, 22.06.2019 23:00
Which of your 24 wells had indications that a chemical reaction occurred? how were you able to tell that a chemical reaction occurred? which of your 24 wells had indications that a physical reaction occurred? how were you able to tell that a physical reaction occurred? report on both mixing and evaporation. make a general statement about whether your hypotheses were validated or rejected. must your hypotheses be correct for this to be a successful laboratory?
Answers: 3


Chemistry, 23.06.2019 12:50
How many energy levels contain electrons in an atom of zirconium (zr)?
Answers: 1
You know the right answer?
Sulfur trioxide (SO3) can be produced using the reversible reaction shown.
Which change would keep...
Questions

Mathematics, 13.04.2020 18:59

Chemistry, 13.04.2020 18:59


Mathematics, 13.04.2020 18:59


English, 13.04.2020 19:00

English, 13.04.2020 19:00

Mathematics, 13.04.2020 19:00


Chemistry, 13.04.2020 19:00



Mathematics, 13.04.2020 19:00

English, 13.04.2020 19:00



Health, 13.04.2020 19:00

Biology, 13.04.2020 19:00


Spanish, 13.04.2020 19:00



