
Chemistry, 18.06.2021 22:00 davisearron
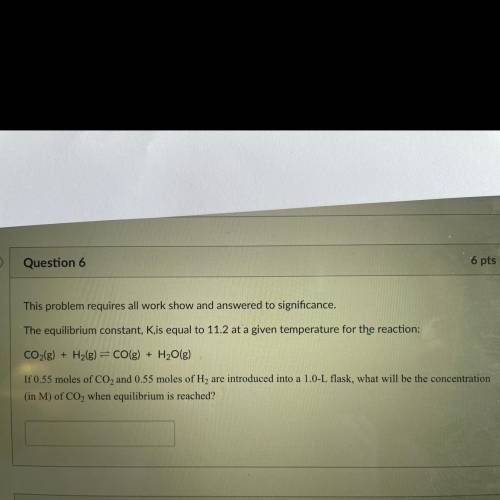
This problem requires all work show and answered to significance.
The equilibrium constant, Kis equal to 11.2 at a given temperature for the reaction:
CO2(g) + H2(g) = CO(g) + H2O(g)
If 0.55 moles of CO2 and 0.55 moles of H2 are introduced into a 1.0-L flask, what will be the concentration
(in M) of CO2 when equilibrium is reached?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 04:20
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1

Chemistry, 22.06.2019 06:30
What is the correct term for living the most sustainable life you can within your current circumstances?
Answers: 1

Chemistry, 22.06.2019 07:30
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2

You know the right answer?
This problem requires all work show and answered to significance.
The equilibrium constant, Kis equ...
Questions


Biology, 03.02.2021 19:20










Mathematics, 03.02.2021 19:20




Mathematics, 03.02.2021 19:20







