
Chemistry, 18.06.2021 22:30 rosenatalie222
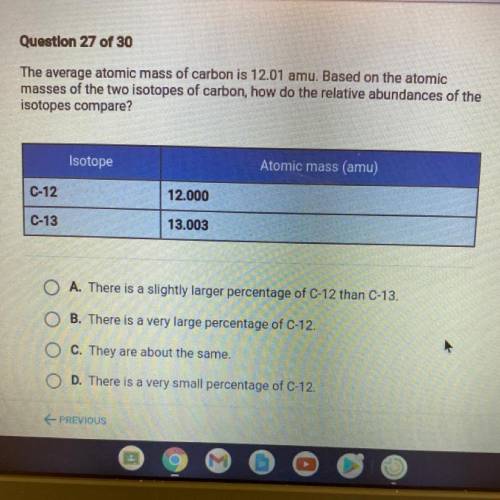
PLS HELP The average atomic mass of carbon is 12.01 amu. Based on the atomic
masses of the two isotopes of carbon, how do the relative abundances of the
isotopes compare?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 21:00
What pressure will be exerted by 0.675 moles of a gas at 25*c if it is in a 0.750-l container?
Answers: 1

Chemistry, 21.06.2019 22:30
Ionic compounds are made of ions, and yet the overall charge of an ionic compound is neutral. why?
Answers: 1

Chemistry, 22.06.2019 02:00
Which of the following is not a good technique for managing used oil? a) have specific, labeled catch pans available for technicians who are collecting oil b) spills in your shop and any releases on pavement or outside should be poured down a drain c) do not use oil containers for antifreeze or other non-similar fluids d) be prepared for oil spills with the proper absorbents
Answers: 1

Chemistry, 22.06.2019 05:40
Consider the elements bromine and chlorine; which elements has a larger ionic radius ?
Answers: 1
You know the right answer?
PLS HELP The average atomic mass of carbon is 12.01 amu. Based on the atomic
masses of the two isot...
Questions

History, 20.05.2021 23:40

Spanish, 20.05.2021 23:40

Mathematics, 20.05.2021 23:40

Chemistry, 20.05.2021 23:40


Mathematics, 20.05.2021 23:40

History, 20.05.2021 23:40

Mathematics, 20.05.2021 23:40

Mathematics, 20.05.2021 23:40

Mathematics, 20.05.2021 23:40

Biology, 20.05.2021 23:50

Mathematics, 20.05.2021 23:50


Biology, 20.05.2021 23:50

Social Studies, 20.05.2021 23:50

Physics, 20.05.2021 23:50




Mathematics, 20.05.2021 23:50



