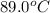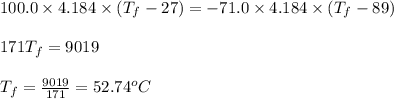Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 17:20
The small bags of silica gel you often see in a new shoe box are placed there to control humidity. despite its name, silica gel is a solid. it is a chemically inert, highly porous, amorphous form of sio2. because water vapor readily adsorbs onto the surface of silica gel, it acts as a desiccant. despite not knowing mechanistic details of the adsorption of water onto silica gel, from the information provided you should be able to make an educated guess about the thermodynamic characteristics of the process. predict the signs for δg, δh, and δs for the adsorption of water.
Answers: 2

Chemistry, 23.06.2019 09:20
Which of the following occurs along coasts during the day?
Answers: 3

Chemistry, 23.06.2019 22:30
What is the thermodynamic driving force for dissolving a solid in a liquid if it is an endothermic process (which reduces the entropy of the surroundings)? 1. the combustion of propane. 2. the increase of the entropy of the system. 3. increased temperature of the system and surroundings. 4. the decrease of the entropy of the system. 5. the increase or decrease of the entropy of the system?
Answers: 1

Chemistry, 24.06.2019 02:00
Drag the tiles to the correct boxes to complete the pairs. match the chemical name of each oxide of phosphorus to its chemical formula. phosphorus monoxide p4o7 diphosphorus tetroxide p2o5 tetraphosphorus heptoxide p2o4 diphosphorus pentoxide po arrowboth arrowboth arrowboth arrowboth
Answers: 1
You know the right answer?
A 100.0-g sample of water at 27.0oC is poured into a 71.0-g sample of water at 89.0oC. What will be...
Questions

English, 25.02.2020 01:00




Mathematics, 25.02.2020 01:00









Mathematics, 25.02.2020 01:01






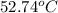
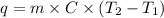
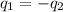
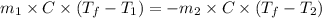 ......(1)
......(1)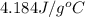
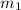 = mass of water of sample 1 = 100.0 g
= mass of water of sample 1 = 100.0 g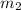 = mass of water of sample 2 = 71.0 g
= mass of water of sample 2 = 71.0 g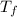 = final temperature of the system = ?
= final temperature of the system = ?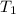 = initial temperature of water of sample 1 =
= initial temperature of water of sample 1 = 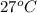
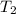 = initial temperature of the water of sample 2 =
= initial temperature of the water of sample 2 =