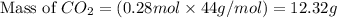Gaseous ethane (CH, CH,) will react with gaseous oxygen (02) to produce gaseous carbon dioxide (CO2) and gaseous water (H,0). Suppose 4.21 g of
ethane is mixed with 31. 9 of oxygen. Calculate the maximum mass of carbon dioxide that could be produced by the chemical reaction. Be sure your answer has
the correct number of significant digits.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:30
The atomic radius of sodium is 186 pm and of chlorine is 100 pm. the ionic radius for na+ is 102 pm and for cl– is 181 pm. in going from na to cl in period 3, why does the atomic radius decrease while the ionic radius increases? a. the inner electrons in the sodium cation shield its valence electrons more effectively than the inner electrons in the chloride anion do. b. the inner electrons shield the valence electrons more effectively in the chlorine atom than in the chloride anion. c. the outermost electrons in chloride experience a smaller effective nuclear charge than those in the sodium cation do. d. the outermost electrons in chloride experience a larger effective nuclear charge than those in the sodium cation do. e. monatomic ions are bigger than the atoms from which they are formed.
Answers: 2


Chemistry, 23.06.2019 01:00
If a sample of radioactive isotopes takes 600 minutes to decay from 400 grams to 50 grams, what is the half-life of the isotope?
Answers: 1

Chemistry, 23.06.2019 11:30
How many grams of carbon are in 237 grams of ethanol(c2h5oh) and how many sulfide ions are in 2.45 moles of aluminum sulfide show me you you got the answers
Answers: 3
You know the right answer?
Gaseous ethane (CH, CH,) will react with gaseous oxygen (02) to produce gaseous carbon dioxide (CO2)...
Questions

Mathematics, 20.10.2019 21:30

Mathematics, 20.10.2019 21:30


Mathematics, 20.10.2019 21:30




Advanced Placement (AP), 20.10.2019 21:30

Mathematics, 20.10.2019 21:30

Mathematics, 20.10.2019 21:30



Health, 20.10.2019 21:30

Mathematics, 20.10.2019 21:30


Mathematics, 20.10.2019 21:30

Chemistry, 20.10.2019 21:30

Chemistry, 20.10.2019 21:30

Biology, 20.10.2019 21:30

Physics, 20.10.2019 21:30

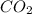 produced is 12.32 g
produced is 12.32 g ......(1)
......(1)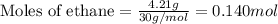
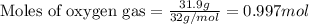
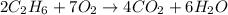
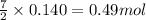 of oxygen gas
of oxygen gas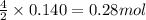 of
of