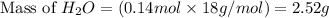Liquid hexane
(CH,(CH), CH) will react with gaseous oxygen (0) to produce gaseous carbon dioxide (CO2) and gaseous water (1,0). Suppose 1.72 g
of hexane is mixed with 8.0 g of oxygen. Calculate the maximum mass of water that could be produced by the chemical reaction. Be sure your answer has the
correct number of significant digits.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:00
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution?
Answers: 2

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 2

Chemistry, 22.06.2019 17:30
Energy defines the different "states" of matter. in no more than 3 sentences, describe the amount of kinetic energy that each of the 3 states of matter possesses and relate that to the atom/molecular motion of each "state".
Answers: 2

You know the right answer?
Liquid hexane
(CH,(CH), CH) will react with gaseous oxygen (0) to produce gaseous carbon dioxide (C...
Questions

History, 28.10.2020 19:30

Biology, 28.10.2020 19:30

Mathematics, 28.10.2020 19:30



Chemistry, 28.10.2020 19:30


Mathematics, 28.10.2020 19:30


Biology, 28.10.2020 19:30

English, 28.10.2020 19:30

Computers and Technology, 28.10.2020 19:30


Physics, 28.10.2020 19:30

Mathematics, 28.10.2020 19:30


Mathematics, 28.10.2020 19:30



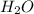 produced is 2.52 g
produced is 2.52 g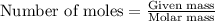 ......(1)
......(1)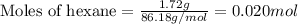

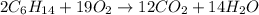
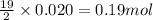 of oxygen gas
of oxygen gas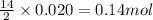 of
of