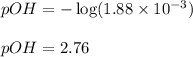Chemistry, 19.06.2021 14:00 pinkycupcakes3oxbqhx
Calculate the pH of a 0.2 M * 4 solution for which Kb = 1.8*10^-5 at 26 c . The equation for the reaction Nh3+H2O->NH4+oh

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 16:00
Silica, sio2, is formed on silicon as an electrically insulating layer for microelectronic devices. silica is formed when silicon is exposed to o2 gas at an elevated temperature. at 900˚c, it takes 90 minutes for the oxygen to diffuse from the surface to form a 0.06 micron (0.06 x 10-6 m) thick layer of sio2 on
Answers: 1

Chemistry, 22.06.2019 05:00
1)each group 16 element has how many valence electrons? ( )4 ( )6 ( )8 ( )16 2)how many dots appear in the dot structure for calcium ion, ca2+? ( )zero ( )one ( )two ( )eight 3) which of the following atoms forms a cation to obtain an octet of outer shell electrons? ( )magnesium ( )oxygen ( )fluorine ( )helium 4) an al3+ ion contains 13 protons and 10 electrons. ( )true ( )false 5) valence and non-valence electrons are represented in lewis dot structures. ( )true ( )false
Answers: 3


Chemistry, 22.06.2019 09:30
1. explain hydrogen peroxide, h 2 o 2 properties and decomposition reaction. 2. describe how each of the following natural cycles plays a part in earth’s climate system. (a) the water cycle (b) the carbon cycle
Answers: 1
You know the right answer?
Calculate the pH of a 0.2 M * 4 solution for which Kb = 1.8*10^-5 at 26 c . The equation for the rea...
Questions


Mathematics, 20.10.2019 00:00

English, 20.10.2019 00:00




Biology, 20.10.2019 00:00




Mathematics, 20.10.2019 00:00


Mathematics, 20.10.2019 00:00

English, 20.10.2019 00:00

Biology, 20.10.2019 00:00

Biology, 20.10.2019 00:00

Chemistry, 20.10.2019 00:00

English, 20.10.2019 00:00


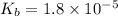
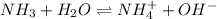
![K_b=\frac{[NH_4^+][OH^-]}{[NH_3]}](/tpl/images/1379/3913/00f50.png)
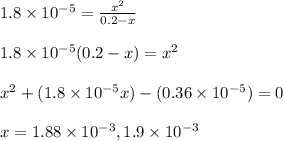
![[OH^-]=x=1.88\times 10^{-3}M](/tpl/images/1379/3913/b0c22.png)
![pOH=-\log [OH^-]](/tpl/images/1379/3913/1fac1.png)